
Safety results in adult upper limb spasticity
Pooled double-blind trials: below are the most common adverse reactions observed in ≥2% of adults with upper limb spasticity (ULS) who received Dysport (up to 1000 Units, n=391) that were reported more frequently than with placebo.1
Swipe the second column to view more information
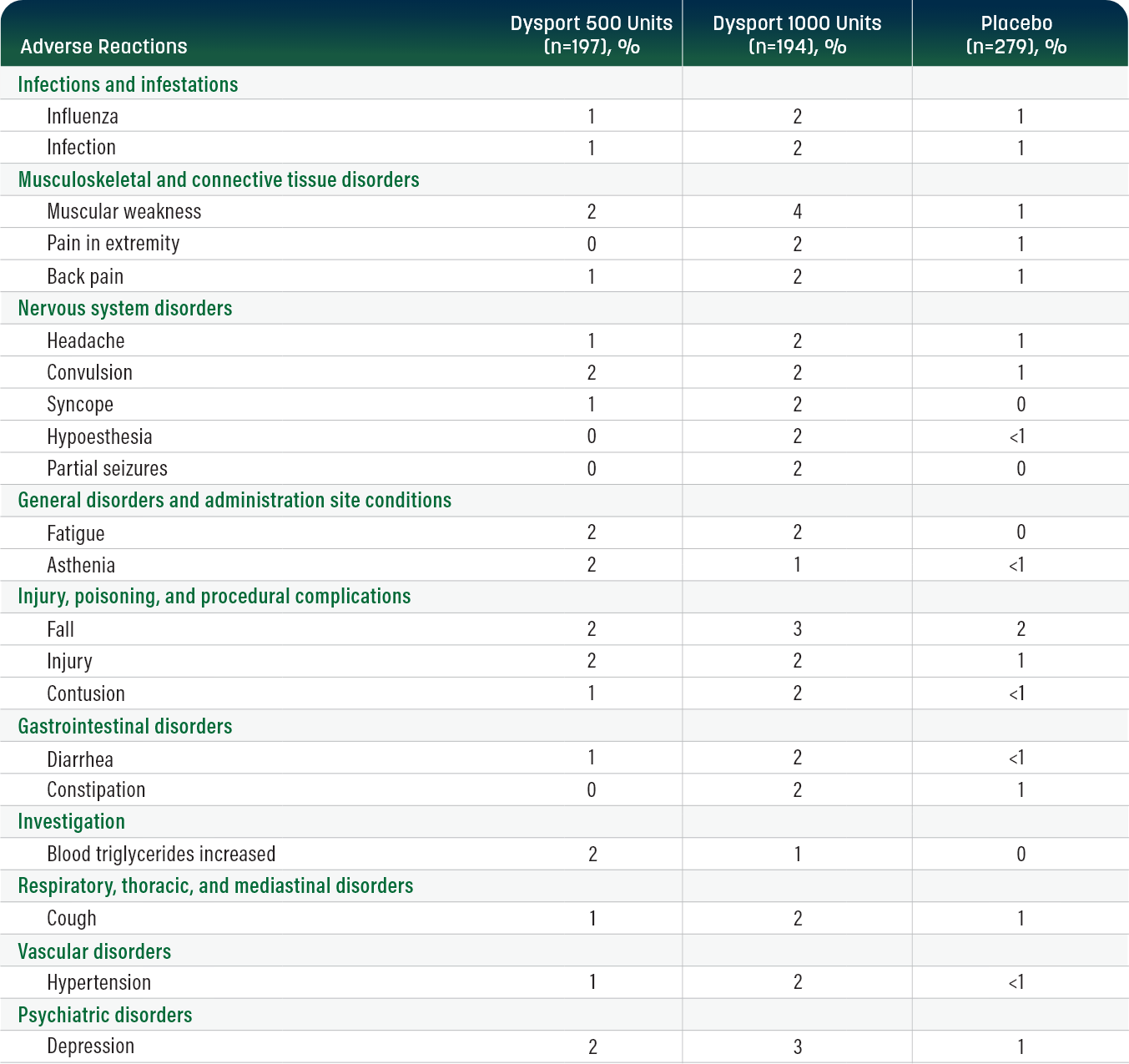
Adverse Reactions

Dysport 500 Units (n=197), %

Dysport 1000 Units (n=194), %
Placebo (n=279), %

In a pooled analysis of clinical studies, adverse reactions with an incidence of less than 2% reported in Dysport treatment groups included dysphagia 0.5%, gait disturbance 0.5%, hypertonia 0.5%, and sensation of heaviness 0.3%.1
Open-label extension: the most commonly observed system organ classes (SOCs) during Cycle 1 were musculoskeletal and connective tissue disorders followed by infections and infestations; general disorders and administration site conditions; and injury, poisoning, and procedural complications. The nature of the most common SOCs and preferred terms (regardless of causality) was similar across all treatment cycles, but the frequency decreased with repeated doses of Dysport. The overall incidence of treatment-emergent adverse events decreased across cycles and was lower in Cycle 2 (27.1%) than in Cycle 1 (40.2%). The corresponding incidence was 26.9% during Cycle 3 and 13.6% during Cycle 4.2
Learn more about Dysport for adult ULS
Safety results in adult lower limb spasticity
Double-blind trial: below are the most common adverse reactions observed in ≥2% of adults with lower limb spasticity (LLS) who received Dysport (up to 1500 Units, n=255) and reported more frequently than with placebo.1
Swipe the second column to view more information
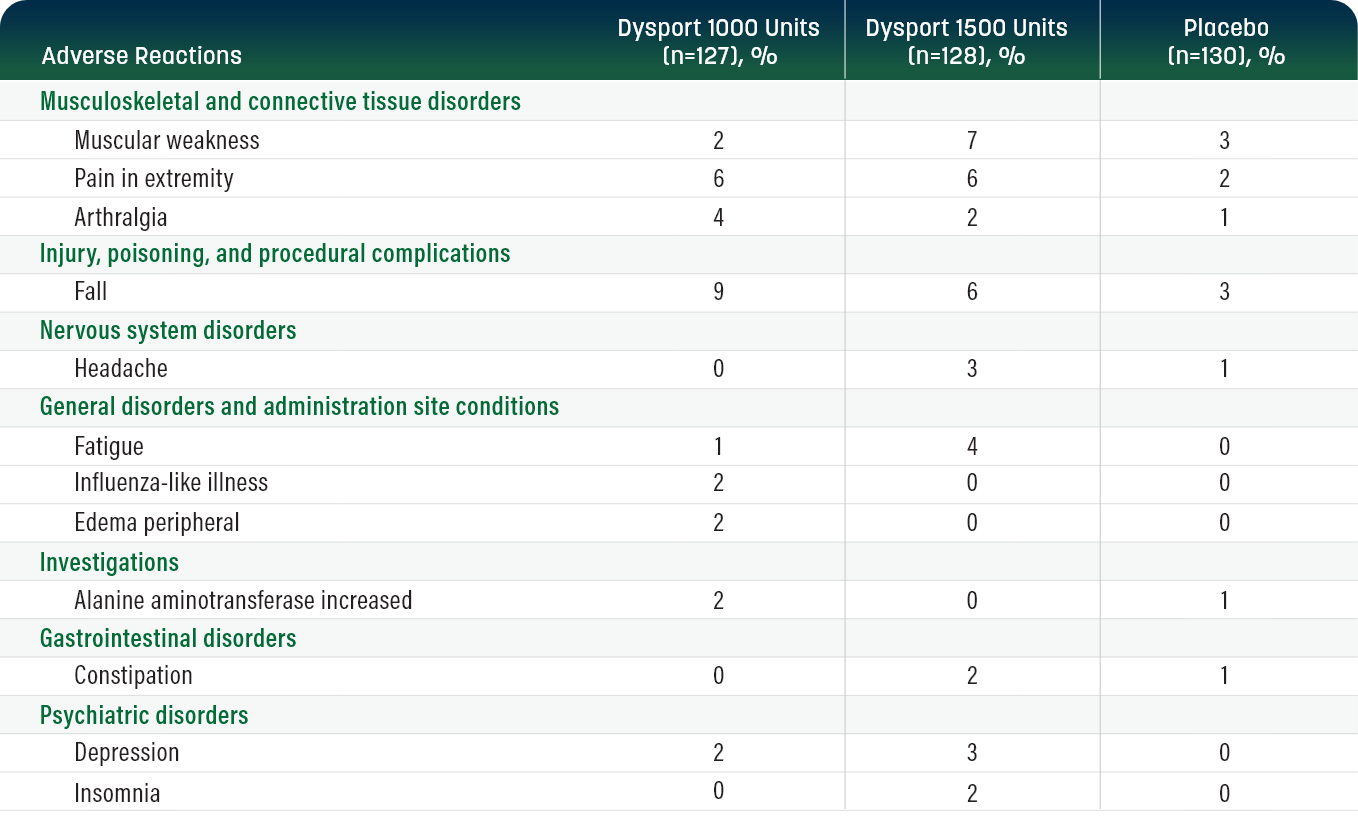
Adverse Reactions

Dysport 1000 Units (n=127), %

Dysport 1500 Units (n=128), %

Placebo (n=130), %

In the efficacy and safety studies of Dysport for the treatment of LLS in adults, muscular weakness was reported more frequently in women (10%) treated with Dysport 1500 Units than in men (5%). Falls were reported more frequently in patients 65 years of age and over.1
In the open-label phase of the study, in subjects only treated in the lower limb with Dysport, fall and muscular weakness were the most commonly reported treatment-emergent adverse events (TEAEs). In subjects treated in the lower limb only, fall was reported in 4.9% of subjects during Cycle 1, 5.7% during Cycle 2, 1.6% during Cycle 3, and 5.6% during Cycle 4. In subjects treated in the lower limb only, muscular weakness was reported in 6.4% of subjects during Cycle 1, 4.0% during cycle 2, 2.4% during Cycle 3, and 1.4% during Cycle 4. Similarly, the most frequently reported TEAEs in subjects who received 500 Units Dysport in the upper limb alongside 1000 Units in the lower limb were fall (7.7% [8/104] of subjects) and muscular weakness (4.8% [5/104]).2
Learn more about Dysport for adult LLS
INDICATIONS
DYSPORT (abobotulinumtoxinA) for injection is indicated for the treatment of:
- spasticity in patients 2 years of age and older
- cervical dystonia in adults
Please see full Prescribing Information, including BOXED WARNING.
IMPORTANT SAFETY INFORMATION
Warning: Distant Spread of Toxin Effect
Postmarketing reports indicate that the effects of DYSPORT and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses and in approved indications, cases of spread of effect have been reported at doses comparable to or lower than the maximum recommended total dose.
Contraindications
DYSPORT is contraindicated in patients with known hypersensitivity to any botulinum toxin products, cow’s milk protein, or to any of the components in the formulation, or infection at the proposed injection site(s). Serious hypersensitivity reactions including anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea have been reported. If such a serious reaction occurs, discontinue DYSPORT and institute appropriate medical therapy immediately.
Warnings and Precautions
Lack of Interchangeability Between Botulinum Toxin Products
The potency Units of DYSPORT are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of DYSPORT cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method.
Dysphagia and Breathing Difficulties
Treatment with DYSPORT and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or swallowing. When distant effects occur, additional respiratory muscles may be involved. Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several weeks and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised. Treatment of cervical dystonia with botulinum toxins may weaken accessory muscles of ventilation, which may result in a critical loss of breathing capacity in patients with respiratory disorders who may have become dependent upon these muscles. Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech, or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin.
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of DYSPORT.
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, vCJD, or CJD have ever been identified for licensed albumin or albumin contained in other licensed products.
Intradermal Immune Reaction
The possibility of an immune reaction when injected intradermally is unknown. The safety of DYSPORT for the treatment of hyperhidrosis has not been established. DYSPORT is approved only for intramuscular injection.
Pre-existing Conditions at the Injection Site
Caution should be exercised when DYSPORT is used where the targeted muscle shows excessive weakness or atrophy.
Adverse Reactions
- The most common adverse reactions (≥4%) in adults with upper limb spasticity include muscular weakness; in adults with lower limb spasticity (≥5%) include falls, muscular weakness, and pain in extremity
- The most common adverse reactions (≥10%) in pediatric patients with upper limb spasticity include upper respiratory tract infection and pharyngitis; in pediatric patients with lower limb spasticity include nasopharyngitis, cough, and pyrexia
- The most common adverse reactions (≥5%) in adults with cervical dystonia include muscular weakness, dysphagia, dry mouth, injection site discomfort, fatigue, headache, musculoskeletal pain, dysphonia, injection site pain, and eye disorders
Drug Interactions
Co-administration of DYSPORT and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents) should only be performed with caution because the effect of the botulinum toxin may be potentiated. Use of anticholinergic drugs after administration of DYSPORT may potentiate systemic anticholinergic effects such as blurred vision. The effect of administering different botulinum neurotoxins at the same time or within several months of each other is unknown. Excessive weakness may be exacerbated by another administration of botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin. Excessive weakness may also be exaggerated by administration of a muscle relaxant before and after administration of DYSPORT.
Please see full Prescribing Information, including BOXED WARNING.
References:
- Dysport® (abobotulinumtoxinA) [Prescribing Information]. Cambridge, MA: Ipsen Biopharmaceuticals, Inc; September 2023.
- Data on file. Cambridge, MA. Ipsen Biopharmaceuticals, Inc.







