
Study design for pediatric upper limb spasticity
The efficacy and safety of Dysport were evaluated in a randomized, prospective, double-blind, multicenter, low-dose controlled, multiple treatment study with pediatric patients aged 2 to 17 with upper limb spasticity (ULS). The study evaluated a total of 208 patients who had a diagnosis of spasticity due to cerebral palsy, were either toxin-naive or non-naive, had a baseline Modified Ashworth Scale (MAS) score of ≥2 in the primary target muscle group (PTMG), and increased muscle tone in at least one upper limb.1,2
The primary efficacy endpoint was mean change from baseline in MAS score in the PTMG at Week 6. Secondary efficacy endpoints were mean change in the Physician Global Assessment (PGA) at Week 6 and mean Goal Attainment Scale (GAS) score at Week 6.1,2
For the first treatment cycle, patients were randomized to receive2:
- Dysport 2 Units/kg (n=69)
- Dysport 8 Units/kg (n=69)
- Dysport 16 Units/kg (n=70)
Patients were reassessed for re-treatment eligibility at Week 16. If ineligible for re-treatment, they were evaluated at every 6 weeks (±2 weeks) until eligible.2
There had to be a minimum of 16 weeks between each injection session, and patients could receive a maximum of 4 sessions over the course of the study. After completing their first treatment cycle, patients receiving Dysport 2 Units/kg were re-randomized to receive Dysport 8 Units/kg or Dysport 16 Units/kg.1,2
Patients receiving the higher doses remained at their dose unless an adjustment up (not exceeding 16 Units/kg) or down was mandated by the investigator. The study remained double-blind for the remaining 3 cycles.2
The PTMG could be changed for the 3 remaining cycles, from elbow to wrist flexors or from wrist to elbow flexors, if the following criteria were met: the MAS score of the new PTMG was higher than the MAS score of the previous PTMG and the new PTMG had a MAS score ≥1.2
Study design for pediatric ULS1,2


PUL – MAS in the PTMG
Dysport significantly reduced muscle tone in high-dose patients1,2


- Patients received Dysport 2 Units/kg, Dysport 8 Units/kg (high-dose), or Dysport 16 Units/kg (high-dose). The primary endpoint was the mean change from baseline in MAS in the PTMG at Week 61
- MAS score at baseline (mean [SD]): Dysport 2 Units/kg, 3.1 (0.3); Dysport 8 Units/kg, 3.1 (0.3); Dysport 16 Units/kg, 3.1 (0.5)1


- Change in MAS in the PTMG was observed through the minimum re-treatment time of 16 weeks
Secondary endpoint was mean PGA score (SD) at Week 61:
- – Dysport 2 Units/kg: 1.7 (0.9), n=69
- – Dysport 8 Units/kg: 2.0 (0.9), n=69
- – Dysport 16 Untis/kg: 2.0 (0.9), n=70
Although PGA scores numerically favored DYSPORT treatment over the low dose control, the difference was not statistically significant.
*Data in the label
ANCOVA=analysis of covariance; CI=confidence interval; ITT=intent-to-treat; LS=least squares; SD=standard deviation.
Sustained relief beyond the minimum time to re-treatment
For many pediatric patients with ULS, the effect of Dysport lasted beyond the minimum 16-week re-treatment period. A majority did not need re-treatment until Week 16 to 28; some experienced an even longer duration of response.1,2


- Data was missing for a total of 9 patients in this group2


Data was missing for a total of 10 patients in this group2
Re-treatment criteria The optimal dose of Dysport, muscles to be injected, and re-treatment eligibility should be selected based on the patient’s progress and response to treatment. In the pivotal trials for pediatric ULS, need for re-treatment was determined by1,2:
- No longer demonstrating a decrease from baseline of ≥1 grade in MAS score in the PTMG
- No improvement in PGA (ie, a score of ≤0)
- No signs of unacceptable safety risk for the next treatment cycle
Eligibility for re-treatment was assessed by the investigator at every visit onward from Week 16 for ULS.2
Repeat Dysport treatment should be administered no sooner than 16 weeks after the previous injection.1
Learn more about Dysport for pediatric ULS
Study design for pediatric lower limb spasticity
The efficacy of Dysport was evaluated in a randomized, prospective, double-blind, multicenter, placebo-controlled study assessing Dysport in pediatric patients aged 2 to 17 with lower limb spasticity (LLS). The study evaluated a total of 235 patients who had a diagnosis of spasticity due to cerebral palsy causing dynamic equinus foot deformity and had a Modified Ashworth Scale (MAS) score of grade ≥2 at the ankle plantar flexors.1,2
Select inclusion criteria included being able to walk with or without walking aids and classified as Gross Motor Function Classification System (GMFCS) Level I to III.2
The co-primary efficacy endpoints were1:
- Reduction in ankle plantar flexor muscle tone at Week 4, as measured by the mean change from baseline in MAS score
- Improvement in response to treatment at Week 4, as measured by mean Physician Global Assessment (PGA) of response to treatment score
Doses of Dysport 10 Units/kg/leg (n=79), 15 Units/kg/leg (n=79), or placebo (n=77) were injected intramuscularly into the gastrocnemius and soleus muscles. The 12-week follow-up visit included assessment for re-treatment eligibility. Patients who remained in the study after Week 12 were permitted additional discretionary follow-up visits at Weeks 16, 22, and 28 to assess eligibility for re-treatment.1,2
Patients eligible for re-treatment were eligible for enrollment into an open-label extension study. In the open-label extension phase, patients received a maximum of 4 treatment cycles at doses of Dysport 5 to 30 Units/kg occurring at intervals of ≥12 weeks. The total dose per cycle was no more than Dysport 30 Units/kg. If physiotherapy or use of casts and/or orthoses was initiated prior to study entry, the therapy regimen continued for the duration of the extension study. If physiotherapy or use of casts and/or orthoses were to be initiated and/or stopped for medical reasons, it was only allowed after Week 12.2
Study design for pediatric LLS1,2


PLL – MAS in ankle plantar flexor
Patients achieved significant reduction in muscle tone1,2


- Dysport significantly reduced MAS score at Week 4 compared to placebo1,2
- MAS score at baseline (mean [SD]): Placebo, 3.2 (0.4); Dysport 10 Units/kg/leg, 3.1 (0.3); Dysport 15 Units/kg/leg, 3.1 (0.3)2
- Co-primary endpoint was the mean change from baseline in MAS in ankle plantar flexor at Week 41
- The investigator graded muscle tone on a 6-point scale, from 0 (no increase in tone) to 4 (affected parts rigid in flexion and extension)2

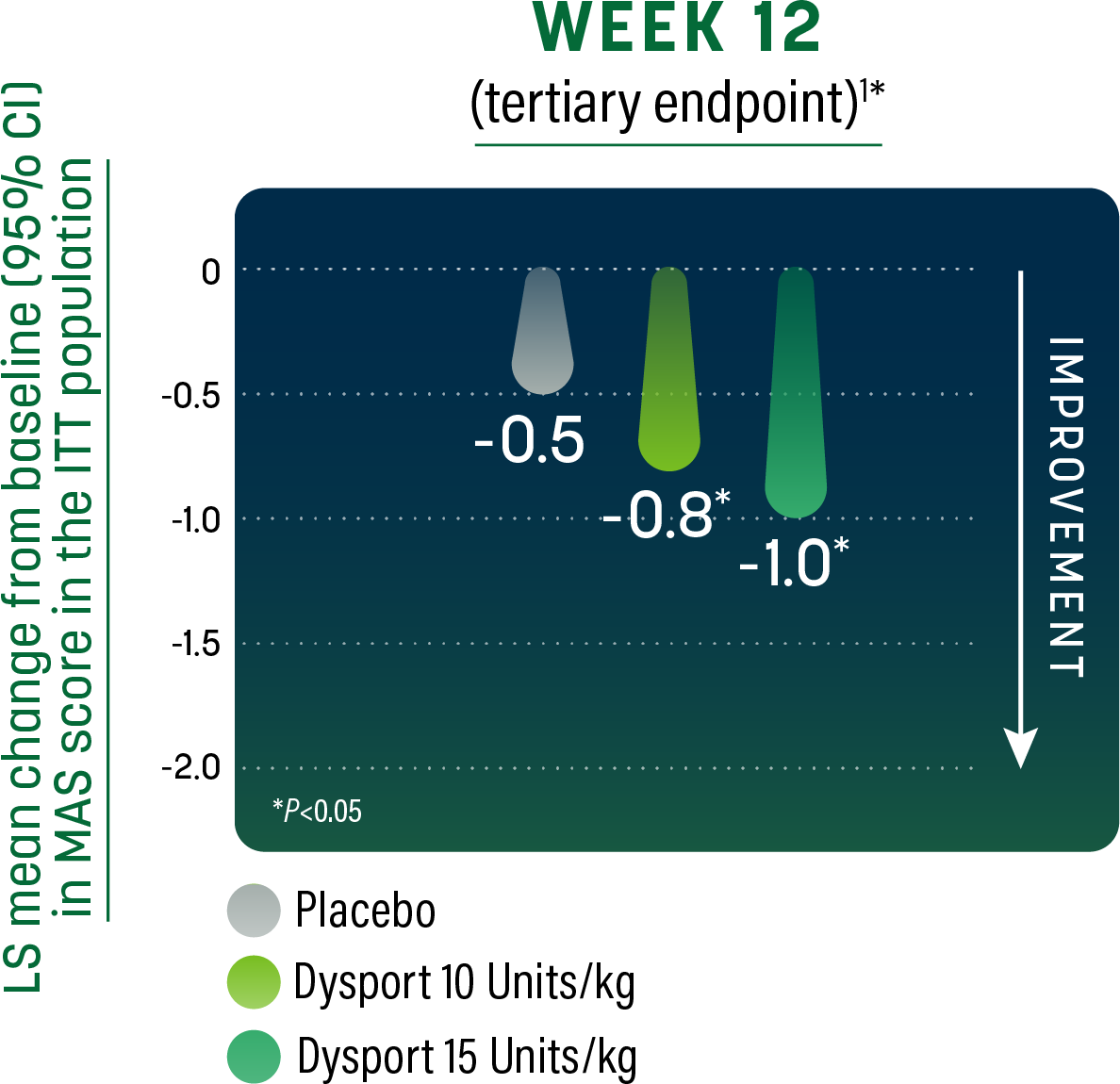
- Significant change in MAS at the ankle plantar flexor was observed through the minimum re-treatment time of 12 weeks1,2
*Data in the label
CI=confidence interval; ITT=intent-to-treat; LS=least squares; SD=standard deviation.
PLL – Physician Global Assessment
Physicians reported a significantly greater response to treatment1


- Patients received Dysport 10 Units/kg, Dysport 15 Units/kg, or placebo. Co-primary endpoint was improvement in response to treatment as assessed by PGA at Week 41
- PGA based on a 9-point rating scale from -4 (markedly worse) to +4 (markedly improved)2


- Significant improvement in response to treatment in patients receiving either dose of Dysport was observed through the minimum re-treatment time of 12 weeks1,2
*Data in the label
Sustained relief beyond the minimum time to re-treatment
For many pediatric patients with LLS, the effect of Dysport lasted beyond the minimum 12-week re-treatment period. A majority did not need re-treatment until Week 16 through Week 22; some experienced an even longer duration of response.1,2
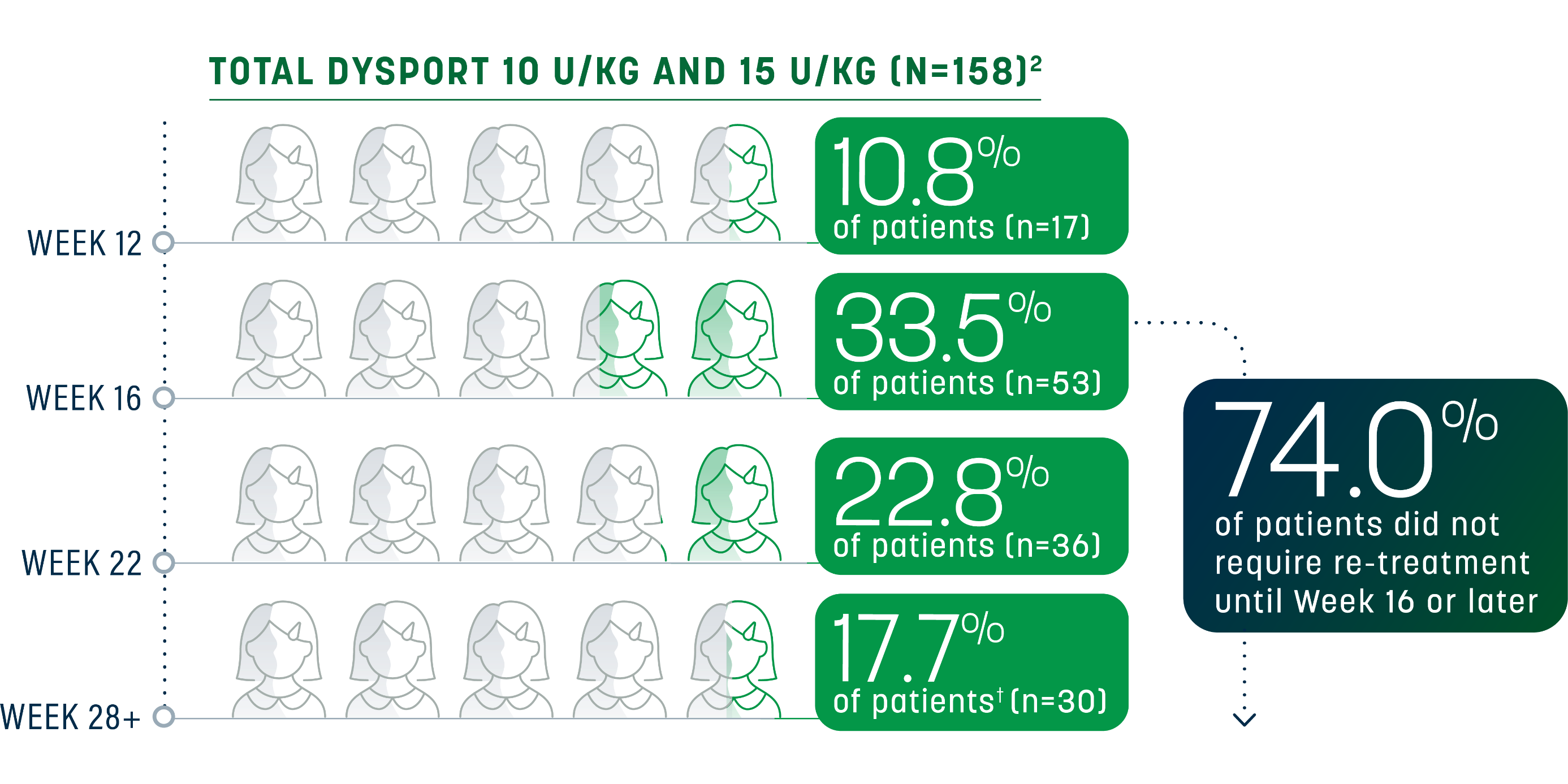

Re-treatment criteria
The optimal dose of Dysport, muscles to be injected, and re-treatment eligibility should be selected based on the patient’s progress and response to treatment. Eligibility for re-treatment was assessed by the investigator at every visit onward from Week 12. Patients who remained in the LLS study after Week 12 were permitted additional discretionary follow-up visits at Week 16, Week 22, and Week 28 to access eligibility for re-treatment. In the pivotal trials for pediatric LLS, need for re-treatment was determined by1,2:
- No longer demonstrating a decrease from baseline of ≥1 grade in MAS score in the ankle flexor
- No improvement in the PGA (score ≤0)
- No signs of unacceptable safety risk for the next treatment cycle
Repeat Dysport treatment should be administered no sooner than 12 weeks after the previous injection.1
Learn more about Dysport for pediatric LLS
INDICATIONS
DYSPORT (abobotulinumtoxinA) for injection is indicated for the treatment of:
- spasticity in patients 2 years of age and older
- cervical dystonia in adults
IMPORTANT SAFETY INFORMATION
Warning: Distant Spread of Toxin Effect
Postmarketing reports indicate that the effects of DYSPORT and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses and in approved indications, cases of spread of effect have been reported at doses comparable to or lower than the maximum recommended total dose.
Contraindications
DYSPORT is contraindicated in patients with known hypersensitivity to any botulinum toxin products, cow’s milk protein, or to any of the components in the formulation, or infection at the proposed injection site(s). Serious hypersensitivity reactions including anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea have been reported. If such a serious reaction occurs, discontinue DYSPORT and institute appropriate medical therapy immediately.
Warnings and Precautions
Lack of Interchangeability Between Botulinum Toxin Products
The potency Units of DYSPORT are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of DYSPORT cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method.
Dysphagia and Breathing Difficulties
Treatment with DYSPORT and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or swallowing. When distant effects occur, additional respiratory muscles may be involved. Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several weeks and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised. Treatment of cervical dystonia with botulinum toxins may weaken accessory muscles of ventilation, which may result in a critical loss of breathing capacity in patients with respiratory disorders who may have become dependent upon these muscles. Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech, or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin.
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of DYSPORT.
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, vCJD, or CJD have ever been identified for licensed albumin or albumin contained in other licensed products.
Intradermal Immune Reaction
The possibility of an immune reaction when injected intradermally is unknown. The safety of DYSPORT for the treatment of hyperhidrosis has not been established. DYSPORT is approved only for intramuscular injection.
Pre-existing Conditions at the Injection Site
Caution should be exercised when DYSPORT is used where the targeted muscle shows excessive weakness or atrophy.
Adverse Reactions
- The most common adverse reactions (≥4%) in adults with upper limb spasticity include muscular weakness; in adults with lower limb spasticity (≥5%) include falls, muscular weakness, and pain in extremity
- The most common adverse reactions (≥10%) in pediatric patients with upper limb spasticity include upper respiratory tract infection and pharyngitis; in pediatric patients with lower limb spasticity include nasopharyngitis, cough, and pyrexia
- The most common adverse reactions (≥5%) in adults with cervical dystonia include muscular weakness, dysphagia, dry mouth, injection site discomfort, fatigue, headache, musculoskeletal pain, dysphonia, injection site pain, and eye disorders
Drug Interactions
Co-administration of DYSPORT and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents) should only be performed with caution because the effect of the botulinum toxin may be potentiated. Use of anticholinergic drugs after administration of DYSPORT may potentiate systemic anticholinergic effects such as blurred vision. The effect of administering different botulinum neurotoxins at the same time or within several months of each other is unknown. Excessive weakness may be exacerbated by another administration of botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin. Excessive weakness may also be exaggerated by administration of a muscle relaxant before and after administration of DYSPORT.
Please see full Prescribing Information, including BOXED WARNING.
References:
- Dysport® (abobotulinumtoxinA) [Prescribing Information]. Cambridge, MA: Ipsen Biopharmaceuticals, Inc; September 2023.
- Data on file. Cambridge, MA. Ipsen Biopharmaceuticals, Inc.










