
Helpful information for ordering Dysport
Each box of Dysport contains 1 sterile, single-use vial with accompanying full Prescribing Information and Medication Guide.


500-Unit Vial
NDC: 15054-0500-01


300-Unit Vial
NDC: 15054-0530-06
Note that for billing purposes, the NDC number requires 11 digits. Therefore, a zero must be entered into the 10th position (eg, 15054-00500-01). This is consistent with Red Book and First Databank Listings.
If you practice within an institution, please acquire Dysport from your wholesalers.
For private practice/clinic, please see below for applicable coverage benefit to acquire Dysport for medical benefit or pharmacy benefit.
Ordering options for Dysport
Medical Benefit
If purchasing Dysport directly (buy and bill):
- Requires upfront financial investment
- Your office acquires Dysport directly from a select group of Specialty Distributors (see list below)
- Your office collects copay/coinsurance directly from patient
- Your office seeks reimbursement from payer(s)
If purchasing from authorized specialty distributors, select from the list below:
Pharmacy Benefit
If Dysport is covered under pharmacy benefit, IPSEN CARES® can help you find a network specialty pharmacy.
- No upfront financial investment
- Patient pays copay/coinsurance directly to Specialty Pharmacy
- Specialty Pharmacy ships Dysport directly to your office
For more information, call IPSEN CARES at 1-866-435-5677, Monday-Friday, 8 AM to 8 PM ET (5 AM to 5 PM PT) or visit IpsenCares.com.
Identifying authentic Dysport for therapeutic use
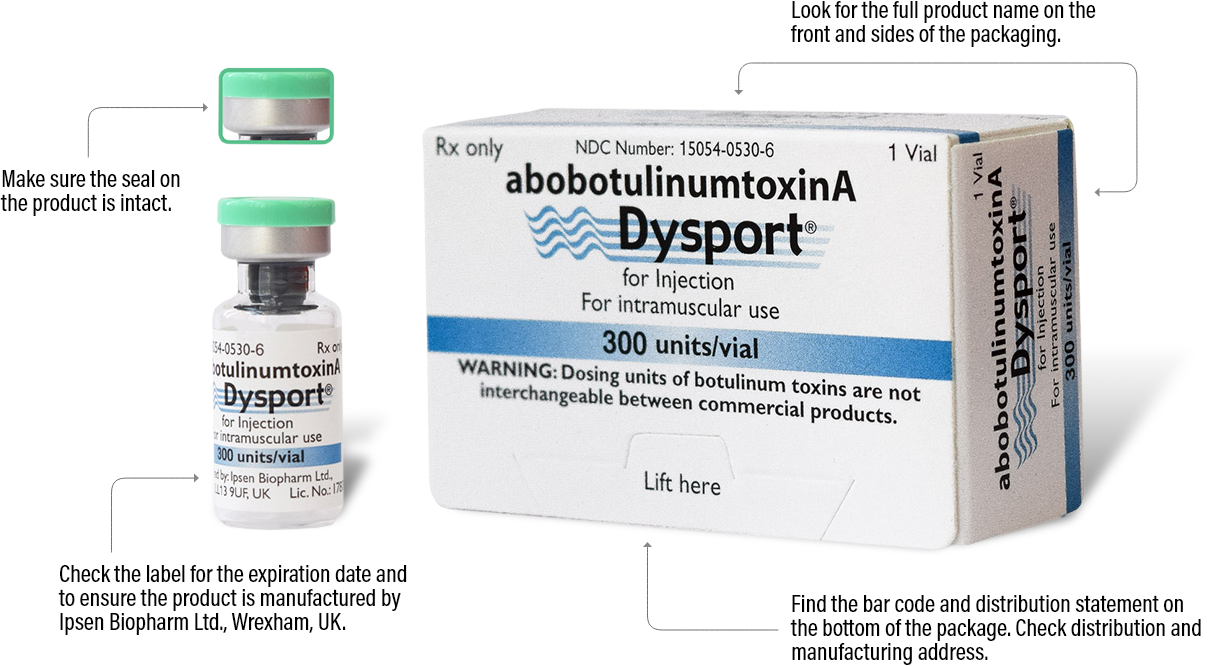
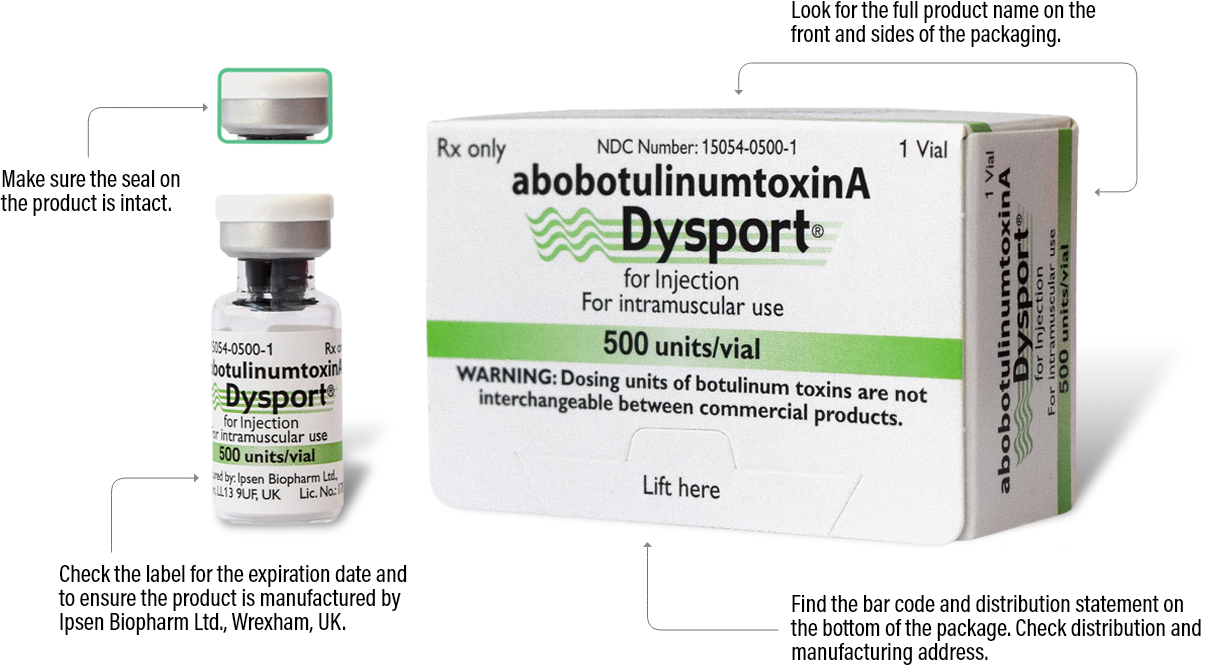
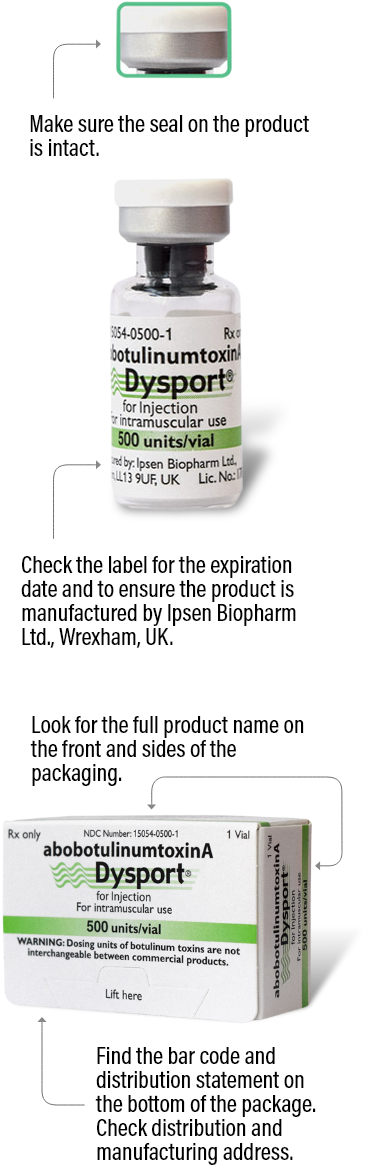
The best way to ensure your patients are receiving authentic Dysport is to order from an authorized Dysport distributor. See below for the various details you can check on each Dysport package to make sure it is authentic.
Please note that there are more anti-counterfeiting measures on the packaging that aren’t listed here. For more information, ask your distributor.
300 units/vial

500 units/vial
Exchange your Dysport vials to extend the shelf life
Through the Dysport Shelf-Life Program you can exchange your Dysport vials with 2 months or less of shelf life with vials that have a longer time until expiration date. To get started:
- Call Ipsen Customer Service at 1-844-94-IPSEN (1-844-944-7736)
- We will walk you through the simple steps for exchange
- Allow at least 10 days from shipment of your short-dated product to receive product that has a longer time to expiration
If we don’t have product available with a longer shelf life when you call for an exchange, Ipsen Customer Service will note your request and notify you when replacement product is available for shipment.
Your request will be prioritized in the order it was made. See below for more details.
Why we created the Shelf-Life Program
Dysport has 24 months of shelf life and is fully usable until the last day of its expiry. However, as a result of the manufacture and distribution of product from Wrexham, UK, to the United States, and the associated Customs, FDA, and other regulatory approvals, product is distributed to Health Care Providers such as yourself with less than 24 months of shelf life.
Conditions for exchange
- Exchanges can only be provided if Ipsen has sufficient product available with a longer shelf life
- Exchanges are capped at a minimum of 2 months of purchase (based on the quality of vials purchased during the most recent rolling 6-month period) every calendar year
- Exchange product must meet all criteria listed on the Ipsen Return Goods Policy
- Exchanges will be provided upon return of short-dated product; Ipsen will provide shipping labels
The Ipsen Return Goods Policy will be in full effect and available should you prefer to receive credit for a qualified return. The Dysport Shelf-Life Program is being offered to customers for a limited time and is subject to change at any time.
Learn more about helping patients access Dysport
INDICATIONS
DYSPORT (abobotulinumtoxinA) for injection is indicated for the treatment of:
- spasticity in patients 2 years of age and older
- cervical dystonia in adults
Please see full Prescribing Information, including BOXED WARNING.
IMPORTANT SAFETY INFORMATION
Warning: Distant Spread of Toxin Effect
Postmarketing reports indicate that the effects of DYSPORT and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses and in approved indications, cases of spread of effect have been reported at doses comparable to or lower than the maximum recommended total dose.
Contraindications
DYSPORT is contraindicated in patients with known hypersensitivity to any botulinum toxin products, cow’s milk protein, or to any of the components in the formulation, or infection at the proposed injection site(s). Serious hypersensitivity reactions including anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea have been reported. If such a serious reaction occurs, discontinue DYSPORT and institute appropriate medical therapy immediately.
Warnings and Precautions
Lack of Interchangeability Between Botulinum Toxin Products
The potency Units of DYSPORT are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of DYSPORT cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method.
Dysphagia and Breathing Difficulties
Treatment with DYSPORT and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or swallowing. When distant effects occur, additional respiratory muscles may be involved. Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several weeks and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised. Treatment of cervical dystonia with botulinum toxins may weaken accessory muscles of ventilation, which may result in a critical loss of breathing capacity in patients with respiratory disorders who may have become dependent upon these muscles. Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech, or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin.
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of DYSPORT.
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, vCJD, or CJD have ever been identified for licensed albumin or albumin contained in other licensed products.
Intradermal Immune Reaction
The possibility of an immune reaction when injected intradermally is unknown. The safety of DYSPORT for the treatment of hyperhidrosis has not been established. DYSPORT is approved only for intramuscular injection.
Pre-existing Conditions at the Injection Site
Caution should be exercised when DYSPORT is used where the targeted muscle shows excessive weakness or atrophy.
Adverse Reactions
- The most common adverse reactions (≥4%) in adults with upper limb spasticity include muscular weakness; in adults with lower limb spasticity (≥5%) include falls, muscular weakness, and pain in extremity
- The most common adverse reactions (≥10%) in pediatric patients with upper limb spasticity include upper respiratory tract infection and pharyngitis; in pediatric patients with lower limb spasticity include nasopharyngitis, cough, and pyrexia
- The most common adverse reactions (≥5%) in adults with cervical dystonia include muscular weakness, dysphagia, dry mouth, injection site discomfort, fatigue, headache, musculoskeletal pain, dysphonia, injection site pain, and eye disorders
Drug Interactions
Co-administration of DYSPORT and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents) should only be performed with caution because the effect of the botulinum toxin may be potentiated. Use of anticholinergic drugs after administration of DYSPORT may potentiate systemic anticholinergic effects such as blurred vision. The effect of administering different botulinum neurotoxins at the same time or within several months of each other is unknown. Excessive weakness may be exacerbated by another administration of botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin. Excessive weakness may also be exaggerated by administration of a muscle relaxant before and after administration of DYSPORT.
Please see full Prescribing Information, including BOXED WARNING.










